UPDATE: FDA Reissues Warning About Sileo Following Additional Overdoses
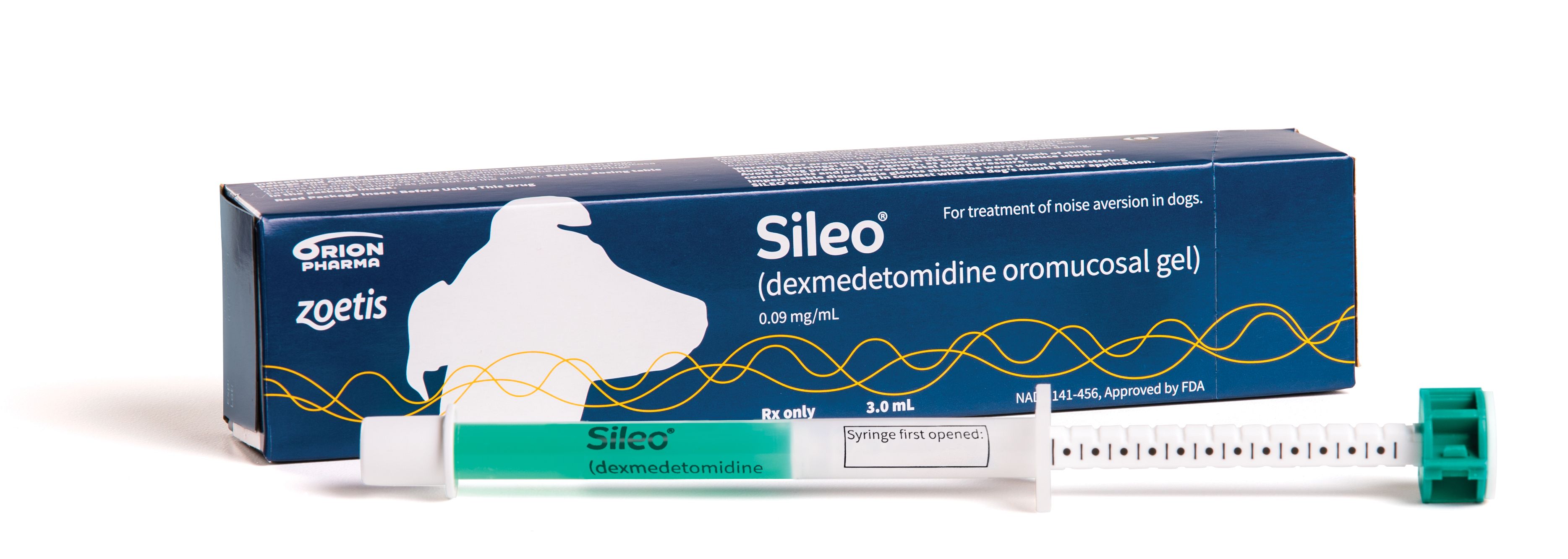
If the oral dosing syringe for Sileo, a drug prescribed for dogs suffering from noise aversion, is not locked properly, the dog may receive an accidental overdose of the drug.
UPDATE (JUNE 29, 2018) — Today, the FDA reissued an advisory that warns veterinarians and pet owners about the risk of accidental overdose for dogs prescribed Sileo. This prescription gel is given to dogs orally for the treatment of noise aversion.
From May 2016—when Zoetis started marketing the product—through May 16, 2018, the FDA has received 54 adverse event reports involving Sileo overdoses in dogs. These overdoses are the result of the product’s ring-stop mechanism not properly locking at the intended dose.
Sileo is packaged in an oral dosing syringe with a ring-stop mechanism on the plunger that must be “dialed” and locked into place to set the correct dose for the dog. Overdose can result if the ring-stop is not fully locked. Therefore, it is very important that the person administering the product understands how to operate the syringe correctly before giving the product to a dog.
Within the year that the FDA published its original Animal Drug Safety Communication on the issue, an additional 26 accidental overdoses in dogs were documented. To date, no deaths have been reported.
The FDA urges all prescribing veterinarians and users to be aware of the possibility for accidental overdose if the syringe is not properly locked before dosing and administering.
(May 2017) — The US Food and Drug Administration is warning veterinarians and pet owners about the potential risk for accidental overdose in dogs prescribed the drug Sileo (dexmedetomidine oromucosal gel), which is marketed by Zoetis—the world’s largest producer of medicine and vaccinations for pets and livestock.
Credit: Zoetis
Sileo is a prescription gel given to dogs orally to treat signs of noise aversion. It is packaged in an oral dosing syringe with a ring-stop mechanism on the plunger that must be locked in place to set the correct dose. If the ring is not locked fully, the dog may receive an accidental overdose.
The FDA has received 28 reports involving Sileo overdoses in dogs due to the ring-stop not locking properly since Zoetis began marketing the drug in May 2016. In 15 of the 28 reports, dogs experienced clinical signs of an overdose—lethargy, sedation, sleepiness, slow heart rate, loss of consciousness, shallow or slow breathing, trouble breathing, impaired balance or incoordination, low blood pressure, and muscle tremors. No deaths have been reported from Sileo overdose.
It is very important for pet owners and veterinarians to understand how to operate the syringe properly before administering the medication. Veterinarians should be aware of the possibility of an accidental overdose and provide proper education to dog owners before prescribing the medication.
Zoetis has provided online resources to demonstrate the proper operation of the Sileo syringe in detail for both veterinarians and dog owners.
UN, WHO address public health concern over avian flu transmission to humans
April 18th 2024Veterinary professionals working with certain animals are advised to take precautionary steps to minimize risk of infection, while researchers in Texas study potential H5N1 vaccines, antivirals, and antibody therapies for humans
Read More